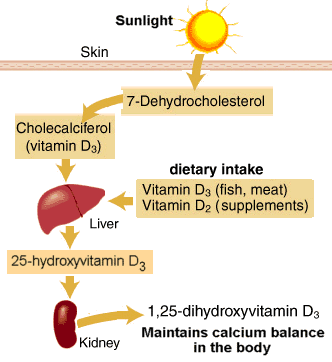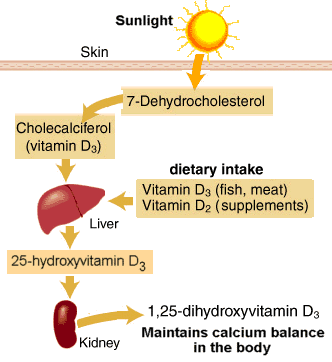
 An interesting study published in Healio Hepatology: “High HBV viral load tied to low serum vitamin D levels” discusses the relationship between the HBV viral load and vitamin D levels. In fact is shows seasonal fluctuations of HBV viral load associated with vitamin D levels. Vitamin D has been on the radar for years, but this interesting correlation between HBV virus flucuations and vitamin D levels warrants additional research to investigate how adequate vitamin D levels can positively impact treatment for those living with chronic HBV. Please refer to earlier blogs, Hepatitis B and Vitamin D and Got HBV? Adding Vitamin D to Your Diet for additional information. As always, please talk to your doctor and have your serum vitamin D levels checked before making any drastic changes to your diet or supplements you may be taking. Don’t forget that vitamin D is the sunshine vitamin, so be sure to keep in mind the impact of the seasons on your levels.
An interesting study published in Healio Hepatology: “High HBV viral load tied to low serum vitamin D levels” discusses the relationship between the HBV viral load and vitamin D levels. In fact is shows seasonal fluctuations of HBV viral load associated with vitamin D levels. Vitamin D has been on the radar for years, but this interesting correlation between HBV virus flucuations and vitamin D levels warrants additional research to investigate how adequate vitamin D levels can positively impact treatment for those living with chronic HBV. Please refer to earlier blogs, Hepatitis B and Vitamin D and Got HBV? Adding Vitamin D to Your Diet for additional information. As always, please talk to your doctor and have your serum vitamin D levels checked before making any drastic changes to your diet or supplements you may be taking. Don’t forget that vitamin D is the sunshine vitamin, so be sure to keep in mind the impact of the seasons on your levels.
Patients with chronic hepatitis B who also were vitamin D deficient had significantly higher HBV DNA levels than patients with adequate vitamin D concentrations in a recent study.
In a retrospective study, researchers measured the serum levels of 25-hydroxyvitamin D (25OHD) in 203 treatment-naive patients with chronic hepatitis B seen between January 2009 and December 2012. Patients with 25OHD levels less than10 ng/mL were considered severely deficient, levels below 20 ng/mL were considered deficient, and levels of 20 ng/mL or greater were considered adequate. Patients’ samples were collected upon initial presentation, except 29 participants whose samples were taken at antiviral therapy initiation.
The mean 25OHD concentration for the cohort was 14.4 ng/mL. Forty-seven percent of participants were considered 25OHD deficient; 34% were severely deficient. 25OHD levels were similar between Caucasians (14.38 ng/mL) and non-Caucasians (14.59 ng/mL) (P=.7).
An inverse correlation was observed between levels of HBV DNA and 25OHD (P=.0003). Multivariate analysis indicated that HBV DNA was strongly predictive of low 25OHD levels (P=.000048), and vice versa (P=.0013). Patients with HBV DNA levels less than 2,000 IU/mL had 25OHD concentrations of 17 ng/mL; those with 2,000 IU/mL or higher had concentrations of 11 ng/mL (P<.00001 for difference). Participants who tested positive for hepatitis B e antigen (HBeAg; n=26) had significantly lower 25OHD levels than HBeAg-negative participants (P=.0013); this association was significant only under univariate analysis.
Investigators also noted fluctuations in HBV DNA and 25OHD levels according to season. Significantly lower HBV DNA levels were observed among samples taken during spring or summer than in autumn or winter (P=.01).
“The present study demonstrates a profound association between higher levels of HBV replication and low [25OHD] serum levels in chronic hepatitis B patients,” the researchers wrote. “At least in patients without advanced liver disease … HBV DNA viral load appears to be the strongest determinant of low [25OHD] serum levels. … Future studies to evaluate a therapeutic value of vitamin D and its analogs in HBV infection may be justified.”