This month, researchers at Jilin University in Changchun, China have discovered an anti-HBV role of the HIV-1 host restriction factor SERINC5. At Seoul National University in South Korea, HBV researchers have elucidated a mechanism by which HBV hijacks host transcription regulation. Researchers from the Paul Ehrlich Institute in Langen, Germany have demonstrated that HBV DNA can be sensed by the cGAS/STING pathway, but is not in the context of natural hepatocyte infection.
- SERINC5 Inhibits the Secretion of Complete and Genome-Free Hepatitis B Virions Through Interfering with the Glycosylation of the HBV Envelope – Frontiers in Microbiology
This paper from Jilin University in Changchun, China reveals the protein serine incorporator 5 (SERINC5) as a host restriction factor for HBV virion secretion. The SERINC family of proteins facilitate lipid biosynthesis and transport in mammalian cells. SERINC5 was recently shown to restrict the replication of HIV-1 and other retroviruses by incorporating into the membrane of budding virions and preventing their entry into target cells. Additionally, the HIV-1 protein NEF as well as the structurally unrelated murine leukemia virus (MLV) protein glycogag have been shown to down-regulate SERINC5 expression on cell surfaces. In this paper, the role of SERINC5 in HBV replication was examined. SERINC5 was found to inhibit HBV virion secretion but not affect intracellular core particle-associated DNA or RNA. Furthermore, the group found that SERINC5 decreased the glycosylation levels of the HBV surface antigens (HBsAg) LHB, MHB, and SHB (large, medium, and small). In order to determine the possible role of SERINC proteins in HBV replication, SERINC proteins 1, 3, and 5, were each transfected into cells alongside an HBV expression vector using Lipofectamine 2000. Transfection of SERINC plasmids was performed in a dose-responsive manner and was confirmed using Western blot. Transfected cell supernatants were then analyzed using an ELISA for HBsAg. Cells transfected with SERINC5 showed a reduction of HBsAg in the supernatant with increasing amounts of SERINC5. Extracellular HBsAg levels in cells transfected with SERINC1 or SERINC3 were unaffected. Furthermore, compared to cells transfected with a control vector, cells transfected with SERINC5 had less HBV virion DNA in the supernatant as measured by qPCR following immunoprecipitation with an anti-HBsAg antibody. Those cells transfected with SERINC1 or SERINC3 showed no change in extracellular HBV virion DNA compared to the control. Interestingly, intracellular levels of HBV DNA and HBV RNA as measured by Southern blot and Northern blot respectively, showed no change between cells transfected with the control vector or any of the SERINC proteins. Additionally, siRNA knockdown of SERINC5 in HepG2 cells concomitantly transfected with an HBV expression vector yielded increased secretion of HBsAg as measured by ELISA and HBV viron DNA as measured by qPCR following immunoprecipitation with an anti-HBsAg antibody. Next, in order to understand the mechanism of SERINC5-mediated HBV secretion inhibition, flag-tagged LHB, MHB, or SHB were transfected into HepG2 cells alongside either a plasmid expressing HA-tagged SERINC5 or a control vector. Interestingly, the glycosylated forms of all three HBsAg proteins were reduced in cells co-transfected with SERINC5 as measured by Western blot. The group then found that SERINC5 colocalizes with LHB in the Golgi apparatus. This was accomplished by co-transfecting HepG2 cells with LHB fused to enhanced cyan fluorescent protein (LHB-ECFP) alongside HA-tagged SERINC5. Cells were then subjected to immunofluorescence dual staining with an antibody against HA as well as an antibody against GM130, a resident protein of the Golgi. These three signals overlapped, implying that SERINC5 interacts with LHB in the Golgi. This finding was further validated by co-immunoprecipitation experiments showing the interaction of SERINC5 with LHB, MHB, and SHB. The group also found, using mutagenesis studies that the fourth to sixth domains of SERINC5 are required for inhibition of HBV secretion. These domains are different than those involved in HIV-1 inhibition, and the group has concluded that SERINC5 inhibits HBV by a completely different mechanism than it does HIV-1. While SERINC5 inhibits HIV-1 by inducing conformational changes on the viral envelope, it inhibits HBV secretion by preventing glycosylation of HBsAg. This publication demonstrates that SERINC5 is a potential anti-HBV host factor. Stimulation of SERINC5 may be a possible treatment for chronic HBV and SERINC5 may prove useful as a diagnostic marker if it is found to correlate with HBV viral load and chronicity.
- Viral hijacking of the TENT4–ZCCHC14 complex protects viral RNAs via mixed tailing – Nature Structural & Molecular Biology
This paper from Seoul National University in South Korea identifies the TENT4-ZCCHC14 complex as a host factor which protects viral messenger RNA (mRNA) transcripts from degradation. Terminal nucleotidyltransferases (TENTs) are noncanonical poly(A) polymerases. These enzymes add many adenine residues as well as occasional non-adenosine residues to the 3′ end of mRNA molecules. TENT4A and TENT4B (also known as PAPD7 and PAPD5) extend mRNA poly(A) tails with the occasional non-adenosine residue which is typically a guanosine. The results are mRNAs bearing “mixed tails”. Deadenylases are enzymes which trim poly(A) tails to initiate mRNA degradation. The carbon catabolite repression 4–negative on TATA-less (CCR4-NOT or CNOT) complex is the main cytoplasmic deadenylase complex. CNOT trims mRNA poly(A) tails, but its activity is hindered when it encounters a guanosine reside. Therefore, mixed tails protect mRNAs from being targeted for degradation. Interestingly, the inhibitor of HBV called DHQ-1 was recently found to interact with TENT4A and TENT4B. The protein called zinc finger CCHC domain-containing protein 14 (ZCCHC14) was previously found to be an essential host factor for HBV surface antigen production in a genome-wide CRISPR screen. This publication demonstrates that ZCCHC14 recognizes a pentaloop motif in the HBV post-transcriptional regulatory element (PRE) of HBV mRNAs and in turn recruits TENT4A or TENT4B which provide the mRNAs with a protective mixed tail. Additionally, it was demonstrated that viral mRNAs of the human cytomegalovirus (HCMV) contain a similar pentaloop motif and also receive protective mixed tails. This group used a method which they developed previously called TAIL-seq. This method allows for sequencing of 3′ tails on mRNAs as well as identification of the transcript. First, total RNA is extracted from cells. Ribosomal RNA (rRNA) is removed using an rRNA depletion kit in which ssDNA probes are specifically bound to rRNA which are then digested by RNase H. Next, a biotinylated adaptor sequence is ligated to the 3′ end of RNAs. A low concentration of RNase T1 is then used to partially digest the transcripts. Next, the RNAs are pulled down, using streptavidin, phosphorylated, and gel purified to obtain fragments which are 500-1000 nucleotides in length. This size fractionation step removes small non-coding RNAs such as tRNA, snRNA, snoRNA, and miRNA. Next, a second adaptor sequence is added to the 5′ end of the mRNAs. Finally, the mRNAs are subjected to next generation sequencing (NGS) on an Illumina HiSeq 2500 platform. Two reads are obtained for each mRNA, one from the 3′ adaptor and one from the 5′ adaptor. Sequence information derived from these reads reveals the specific composition of mRNA poly(A) tails. In this publication, TAIL-seq was employed to investigate viral mRNA tailing. HepG2.2.15 cells which express the HBV genome, as well as human foreskin fibroblasts (HEF) infected with HCMV were subjected to TAIL-seq. mRNA 3′ tails of both viruses were found to be guanylated significantly more than cellular mRNAs. Additionally, viral mRNA 3′ tails were longer than cellular ones, indicating slower net deadenylation. To check the mechanism of viral mixed tailing, the noncanonical poly(A) polymerases TENT4A and TENT4B were knocked down using siRNA. TAIL-seq showed a significant reduction of viral mRNA 3′ tail guanylation in TENT4-knockdown cells. Additionally, the half-lives of HBV mRNAs were shown to decrease in TENT4-knockdown HepG2.2.15 cells as measured by RT-qPCR at intervals following the addition of the transcription blocker actinomycin D. In order to determine how HBV mRNAs recruit TENT4A and TENT4B, formaldehyde-based crosslinking and immunoprecipitation sequencing (fCLIP-seq) was employed on HepG2.2.15 cells. fCLIP-seq reveals what RNA sequences proteins bind to. In fCLIP-seq, formaldehyde is used to crosslink RNA-protein interactions. RNA-protein complexes are then “pulled down” using an antibody and run on a gel. The protein may then be degraded using proteinase K and RNA molecules may be sequenced. RNA sequencing reads from fCLIP-seq of the HBV genome were enriched in lysates pulled down using antibodies against TENT4A or TENT4B compared to input cell lysate and that pulled down using normal mouse IgG. Importantly, the greatest enrichment occurred specifically in the PRE region of HBV mRNAs. The group goes on to show that the sterile alpha motif (SAM) of ZCCHC14 binds to the stem loop ⍺ region of the PRE and recruits TENT4 proteins. This publication demonstrates that both HBV and HCMV have taken advantage of host mRNA transcription regulation to prolong transcript half-life. ZCCHC14, TENT4A, and TENT4B may be possible host targets for HBV or HCMV antiviral treatments.
- Hepatitis B Virus DNA is a Substrate for the cGAS/STING Pathway but is not Sensed in Infected Hepatocytes – Viruses This paper from the Paul Ehrlich Institute in Langen, Germany shows that HBV DNA is sensed by cGAS, but not in natural HBV infection of hepatocytes. Cyclic guanosine monophosphate-adenosine monophosphate (cGAMP) synthase (cGAS), is a pattern recognition receptor (PRR) that senses cytoplasmic double-stranded DNA (dsDNA). In response to dsDNA binding, cGAS catalyzes the production of 2’3′-cGAMP, a cyclic dinucleotide (CDN) which activates stimulator of interferon genes (STING) by direct binding. Once activated, STING signaling results in the activation of transcription factors promoting the production of type I interferons (IFN-I) and proinflammatory cytokines including tumor necrosis factor alpha (TNFα). IFN-I production and secretion lead to the activation of numerous IFN-stimulated genes (ISGs) which induce a robust antiviral state in the cell. The cGAS/STING pathway is a key component of innate immunity, protecting cells from bacterial and viral infections. How viruses interact with host innate immune sensors such as cGAS is important for understanding their pathogenesis. While the innate immune mechanisms activated by HBV infection remain disputed, HBV is largely considered to be a stealth virus in that it bypasses host innate immunity. Some groups have postulated that the HBV X protein (HBx) or HBV polymerase may inhibit innate immune responses. In this publication it is demonstrated that HBV RNAs are not immunostimulatory, however HBV DNA does elicit an innate immune response mediated by the cGAS/STING pathway. In order to test the immunostimmulatory potential of HBV nucleic acids, they were transfected at multiple concentrations into monocyte-derived dendritic cells (MDDCs) generated from primary human peripheral blood mononuclear cells (PBMCs). Following transfection, mRNA of the gene ISG54 was measured by RT-qPCR. ISG54 was selected as the read-out for innate immune signaling because it is a direct target of the transcription factor IRF3 which is activated downstream of both RIG-I (RNA-sensing) and cGAS/STING (DNA-sensing) pathways. HBV nucleic acids were extracted from HBV virions and quantified prior to transfection. Some groups of nucleic acids were subjected to either DNase or RNase digestion, leaving only HBV RNA or DNA respectively. Total HBV nucleic acids stimulated ISG54 transcription in a dose-dependent manner. Similarly, HBV DNA also stimulated ISG54 transcription. However, transfection of HBV RNA alone did not activate ISG54 transcription, implying that only HBV DNA elicits an innate immune response. In order to test which specific innate immune pathway senses HBV DNA, the human monocytic leukemia cell line THP-1 was used. CRISPR/Cas9 genome editing was used in THP-1 cells to knockout (KO) cGAS, STING, or mitochondrial antiviral-signaling protein (MAVS), which is a key node downstream of the RNA-sensing RIG-I-like receptor (RLR) protein family. Transfection with HBV nucleic acids caused a high level of ISG54 transcription in wild type (WT) and MAVS KO cells which was abrogated when HBV nucleic acids were treated with DNase prior to transfection. However, HBV nucleic acids caused no measurable ISG54 transcription in either cGAS KO or STING KO cells. Next, the group wanted to determine if HBV activates the cGAS/STING pathway in its natural infection of hepatocytes. The levels of cGAS, STING, and other PRRs in a panel of cells were determined using RT-qPCR. The hepatocellular carcinoma cell line HepG2 as well as primary human hepatocytes (PHH) were shown to express less cGAS and STING than Kupffer cells, MDDCs, THP-1 cells, or monocyte derived macrophages (MDMs). Next, HepG2 cells expressing the human sodium taurocholate cotransporting polypeptide used for HBV cell entry (HepG2-hNTCP) and PHHs were transfected with HBV nucleic acids. Both hepatocyte types showed a dose-responsive increase in ISG54 transcription when transfected. Finally, HepG2-hNTCP cells and PHHs were infected with HBV and HBV RNA and ISG54 mRNA were quantified by RT-qPCR. Although both cell types were efficiently infected, they showed no induction of ISG54 across several days. These results indicate that although hepatocytes are capable of sensing transfected HBV genomic DNA via cGAS, they are not able to do so in the context of a natural infection. One possible explanation for the failure of hepatocytes to sense HBV nucleic acids is that they are shielded by the viral nucleocapsid upon infection and during the formation of replication intermediates. Another possibility is that the level of HBV nucleic acids in a natural infection is too low to activate cGAS/ STING, given that these proteins are sparse in hepatocytes. This publication demonstrated for the first time that HBV RNAs are not immunostimulatory, while HBV DNAs activate the cGAS/STING pathway. This finding shows that it may be possible to utilize the cGAS/STING pathway in order to eradicate chronic HBV infection. Perhaps small molecules which destabilize HBV nucleocapsids may be used to expose the DNA of intracellular HBV virions, leading to the activation of the cGAS/STING pathway and an innate antiviral response.
Meet our guest blogger, David Schad, B.Sc., Junior Research Fellow at the Baruch S. Blumberg Institute studying programmed cell death such as 


 excited to partner with the
excited to partner with the 
 disease.1,3. Additionally; lack of hepatitis B vaccination recommendations for high risk groups, low implementation of hepatitis B screening during pregnancy, supply shortages and vaccine hesitancy, have created opportunities for hepatitis B and D transmission. Exposure to infected blood or sexual fluids through blood transfusions or surgeries (before the 1990’s), tattoos, piercings, injection drug use, or sexual contact with an infected person, can expose people already living with hepatitis B to hepatitis D, or expose those who have not received the full hepatitis B vaccine series to both viruses. Control of hepatitis B and D coinfection has also been hindered by the lack of a national registry and surveillance system thus preventing an understanding of the accurate prevalence and public health burden1.
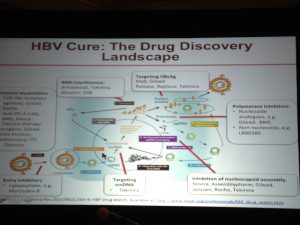
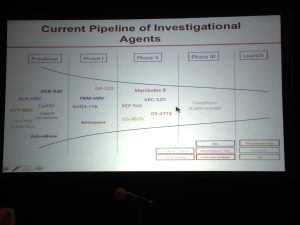
disease.1,3. Additionally; lack of hepatitis B vaccination recommendations for high risk groups, low implementation of hepatitis B screening during pregnancy, supply shortages and vaccine hesitancy, have created opportunities for hepatitis B and D transmission. Exposure to infected blood or sexual fluids through blood transfusions or surgeries (before the 1990’s), tattoos, piercings, injection drug use, or sexual contact with an infected person, can expose people already living with hepatitis B to hepatitis D, or expose those who have not received the full hepatitis B vaccine series to both viruses. Control of hepatitis B and D coinfection has also been hindered by the lack of a national registry and surveillance system thus preventing an understanding of the accurate prevalence and public health burden1. which has led to late diagnoses and cost many patient lives. For patients who are diagnosed, investigational testing is not covered by the national insurance house, placing a financial burden on patients to pay out of pocket for the additional testing necessary to manage their coinfection. With pegylated interferon injections as the only semi-effective treatment option, even diagnosed patients struggle to effectively control their coinfection and even less are connected to clinical trials. Although there are 7 new drugs in clinical trials, progress has lagged behind patient need for new therapies, many of whom are living with cirrhosis.
which has led to late diagnoses and cost many patient lives. For patients who are diagnosed, investigational testing is not covered by the national insurance house, placing a financial burden on patients to pay out of pocket for the additional testing necessary to manage their coinfection. With pegylated interferon injections as the only semi-effective treatment option, even diagnosed patients struggle to effectively control their coinfection and even less are connected to clinical trials. Although there are 7 new drugs in clinical trials, progress has lagged behind patient need for new therapies, many of whom are living with cirrhosis.