
2022 is shaping up to be a big year for hepatitis delta, the rare but serious virus that can co-infect people who are already living with hepatitis B. As a quick refresher, hepatitis delta is a virus that depends upon the hepatitis B virus in order to survive and replicate – so only those who are already living with hepatitis B can become infected with hepatitis delta. Hepatitis delta virus (HDV) is believed to infect between 5 and 10% of people living with hepatitis B virus (HBV). HDV can occur through either a superinfection or a coinfection. A superinfection occurs when someone who is already living with HBV contracts HDV, in which case there is a very high chance that the individual will develop chronic (lifelong) infections of both HBV and HDV. A coinfection occurs when both HBV and HDV are contracted at the same time – when this happens in adults, both infections tend to clear within six months and there is only a 5% chance that chronic HBV and HDV will occur. Chronic HDV is particularly dangerous because it advances progression to serious liver damage and liver failure much more quickly than HBV alone – 70% of people diagnosed with HDV and HBV will experience serious liver damage within 10 years without intervention, compared to 15-30% of people diagnosed with HBV alone.
So, What’s Happening in the World of Hepatitis Delta?
The past 18 months have been very important for hepatitis delta research and drug development. In July of 2020, the European Medicines Agency approved Hepcludex, the first-ever drug approved for treatment of hepatitis delta, for prescription in France, Austria, and Germany. Hepcludex works by stopping HDV from entering and infecting liver cells (and is known as an entry inhibitor). In 2021, MYR Pharma, the German company that originally developed Hepcludex, was bought by Gilead Sciences, Inc., which is based in the United States, and which has since filed a Biologics Licensing Agreement for approval of Hepcludex by the US Food and Drug Administration, which is expected later this year. At this time, there is not a timeline for when Hepcludex approval will be expanded to more countries and parts of the world. Prior to Hepcludex, the only drug available for hepatitis delta management, which was never officially approved, was called pegylated interferon alpha. This drug, still in use today, is only effective in controlling HDV in about 25% of people living with the virus and has challenging side effects that can negatively impact quality of life.
In addition to Hepcludex, two other promising drugs are in clinical trials, both developed by Eiger BioPharma in the United States. The first of these is called Lonafarnib, which is being evaluated for how well it works to target the protein assembly process, which keeps new viruses from being created (it is known as a prenylation inhibitor). Lonafarnib, in combination with another drug called Ritonavir, is currently in Phase III clinical trials (the phase in which the safety and effectiveness of a drug is compared to that of currently available treatments). These trials are fully enrolled, and data is expected by the end of 2022. Additionally, Eiger is currently enrolling phase III clinical trials for Pegylated Interferon Lambda, which works by stimulating the body’s own immune system to fight the virus. For a full list of drugs under investigation for hepatitis delta, including one from Janssen Research and Development and one from Antios Therapeutics, visit our Drug Watch page.
Are There Other Clinical Trials Happening for Hepatitis Delta?
Yes! There are clinical trials happening worldwide to test many of the drugs listed above and more. You can check out our clinical trials page here. This page includes a detailed description of each clinical trial, along with information about where it is being conducted and how to contact the principal investigator (or person leading the clinical trial). This page also includes a helpful graphic describing the clinical trial process and what it takes for a drug to move from an idea into the real world. It is important to note that not all of the trials listed here are for the purpose of testing a medication – some are observational studies to monitor what are called disease biomarkers, which are physical measures used to monitor the progress of a disease and could include tests of blood or liver function, for example. Clinical trials are currently happening in Australia, Austria, Belgium, Brazil, Bulgaria, Canada, China, France, Georgia, Germany, Greece, Israel, Italy, Japan, Mongolia, New Zealand, Pakistan, Republic of Moldova, Romania, Russian Federation, Spain, Sweden, Switzerland, Taiwan, Turkey, Ukraine, the United Kingdom, the United States, and Vietnam.
When Will HDV Drugs and Clinical Trials Be More Accessible in More Parts of the World?
This is unfortunately a difficult question to answer. Even though up to 10% of people who are living with hepatitis B are also living with hepatitis delta, there are not good systems in place to make sure that everyone who is living with HBV or who is at increased risk for HDV is tested and diagnosed, so there are not very accurate numbers about how many people in the world are living with HDV. Indeed, of the nearly 300 million people around the world who are living with hepatitis B alone, only 10% are aware of their diagnosis, so this number is undoubtedly far lower than even 10% for hepatitis delta. Without accurate information about how many people are living with the virus, it is difficult for drug and clinical trial developers to invest resources into studying or pursuing drug development or clinical trials for HDV.
Another problem is the many resources of time, money, and labor that are necessary for developing drugs, and preparing and running clinical trials. The development process for a single drug can take anywhere from 5-15 years and a much larger number of drugs fail to complete this process than succeed. Additionally, there needs to be some degree of existing infrastructure in a particular country in order to both support a clinical trial and ultimately to get a drug approved. Unfortunately, this kind of infrastructure is generally already established and easier to navigate in wealthier countries, so these are the countries in which clinical trials are generally held and in which drug approvals tend to happen first. Public health and clinical infrastructure is slowly developing and becoming more prioritized in different parts of the world and hopefully this trend will continue, but for the time being, the locations of clinical trials and approvals for important treatments point to the much larger issues of lack of access to health and healthcare in much of the world, that in turn stem from deep-seated poverty and inequity. Again, as health equity continues to be a focus of the public eye, these trends will hopefully begin to change, paving the way for greater access to healthcare for hepatitis delta, hepatitis B, and countless other health conditions.
What Is Hep Delta Connect’s Role?
This year, Hep Delta Connect will continue its work to raise the profile of hepatitis delta, both in the United States and around the world. We are committed to building awareness through partnerships with community-based organizations, healthcare providers, and governmental agencies around the world and through dissemination of educational materials and programming. We hope to foster greater engagement of those living with and affected by hepatitis delta globally, more focused advocacy efforts to bring HDV into the spotlight, and increased screening, diagnosis, and management of HDV. We keep our website and social media channels updated regularly with program news and events – make sure to follow us on Facebook, Twitter, and Instagram and check out our website frequently! You are always welcome to connect with us anytime at connect@hepdconnect.org. We look forward to an exciting year of work on HDV!





 NORD through participating in rare disease Twitter chats and presenting a poster at the NORD Rare Action Summit in October 2018. We’re very excited to be a part of the coalition, and to be spreading awareness about hepatitis delta!
NORD through participating in rare disease Twitter chats and presenting a poster at the NORD Rare Action Summit in October 2018. We’re very excited to be a part of the coalition, and to be spreading awareness about hepatitis delta!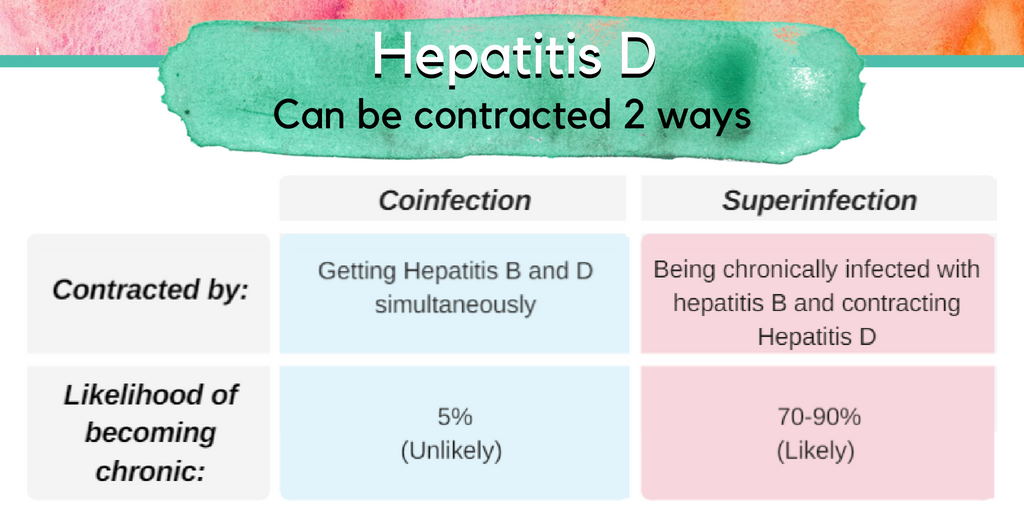 By Sierra Pellechio, Hepatitis Delta Connect Coordinator
By Sierra Pellechio, Hepatitis Delta Connect Coordinator


