 North American Occupational Health and Safety Week (May 5-11) is a time to raise awareness about the importance of injury and illness prevention in the workplace! This week, we’re focusing on health and safety within the nail salon industry, specifically the risk for hepatitis B transmission and opportunities to increase awareness and education about hepatitis B among nail salon workers.
North American Occupational Health and Safety Week (May 5-11) is a time to raise awareness about the importance of injury and illness prevention in the workplace! This week, we’re focusing on health and safety within the nail salon industry, specifically the risk for hepatitis B transmission and opportunities to increase awareness and education about hepatitis B among nail salon workers.
In the U.S., the nail salon workforce is comprised mostly of Vietnamese Americans, with many being immigrants. Refugee and immigrant communities are often susceptible to worker exploitation (including labor trafficking) and encounter cultural and linguistic barriers that may leave them vulnerable to occupational health and safety risks, including hepatitis B transmission.
During routine work, nail technicians may be exposed to a client’s blood or other bodily fluids. It is important for nail salon workers to take precautionary measures to protect themselves and their clients to prevent the potential spread of the hepatitis B virus. More importantly, the nail salon industry (including salon owners and state health departments or boards that regulate nail salons) should implement policies that support greater education, awareness, and prevention of hepatitis B transmission among its workforce.
In October of 2011, the American College of Gastroenterology urged the need for increased surveillance and information on disinfection and infectious disease prevention, particularly for hepatitis B and C in nail salons. Since then, no major research or analysis has been conducted to better understand hepatitis B transmission or the policies that protect nail salon workers. In a new report released by the Hepatitis B Foundation, “The Impact of Nail Salon Industry Policies and Regulations on Hepatitis B Awareness and Prevention,” we seek to further understand the nail salon industry landscape through analyzing state policies that govern nail salons and identify strategies to support increased hepatitis B education, awareness, and prevention.
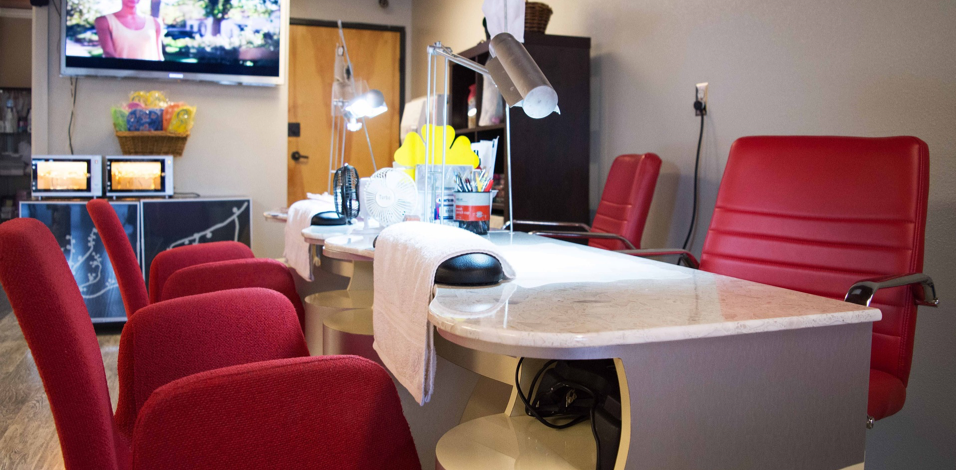 The nail salon industry is regulated at the state level by a regulatory Board of Cosmetology that oversees and ensures nail technicians and nail salons comply with all rules and regulations. In this report, we analyze the nail salon workforce and industry regulations and provide recommendations that can address specific concerns. We conducted phone interviews with health clinics, public health workers, and other relevant stakeholders to better understand the challenges this population encounters when accessing hepatitis B education and care. In addition, we conducted a policy analysis of each state’s Board of Cosmetology to assess their effectiveness in protecting workers from exposure to bloodborne pathogens, specifically hepatitis B. In our analysis, we found that several states may not adequately protect workers from workplace hazards that may increase their risk of hepatitis B exposure. With sanitation and disinfection requirements that greatly vary between states, low compliance can leave workers susceptible to the transmission of bloodborne pathogens, including the hepatitis B virus.
The nail salon industry is regulated at the state level by a regulatory Board of Cosmetology that oversees and ensures nail technicians and nail salons comply with all rules and regulations. In this report, we analyze the nail salon workforce and industry regulations and provide recommendations that can address specific concerns. We conducted phone interviews with health clinics, public health workers, and other relevant stakeholders to better understand the challenges this population encounters when accessing hepatitis B education and care. In addition, we conducted a policy analysis of each state’s Board of Cosmetology to assess their effectiveness in protecting workers from exposure to bloodborne pathogens, specifically hepatitis B. In our analysis, we found that several states may not adequately protect workers from workplace hazards that may increase their risk of hepatitis B exposure. With sanitation and disinfection requirements that greatly vary between states, low compliance can leave workers susceptible to the transmission of bloodborne pathogens, including the hepatitis B virus.
We offered the following recommendations to provide industry changes and community initiatives that can help protect workers or link them to care:
- Build partnerships between community organizations and nail salons to increase hepatitis B education, testing, and vaccination among nail salon workers
- Integrate hepatitis B education into the nail technician licensing curriculum
- Implement continuing education (CE) requirements around hepatitis B prevention and uphold sanitation requirements
- Provide multilingual course training materials and written licensing exams
- Adopt a sanitation rating system
Additionally, through our analysis, we found that four states have policies that discriminate against nail salon workers affected by hepatitis B by barring them from working in nail salons. Even with federal legal protections from the Americans with Disabilities Act, the continued discrimination in this industry presents a clear need to increase hepatitis B knowledge and awareness. Further state-level advocacy will be needed to address discriminatory policies. We must hold states accountable and advocate for policies and regulations that protect individuals affected by hepatitis B and prevent transmission of hepatitis B in the nail salon workplace.

Be sure to check out our full report for a detailed analysis of current state regulations and policies to assess their impact on educating and protecting nail salon workers and preventing hepatitis B transmission in the workplace.
Whether you work in a nail salon or visit one for a manicure or pedicure, be knowledgeable about the steps you can take to protect yourself. For further information about nail salon hazards and a complete guide to protecting your health and preventing injury in the workplace, check out OSHA’s guide here.


 Phase 3 clinical trials have been announced for two drugs,
Phase 3 clinical trials have been announced for two drugs, 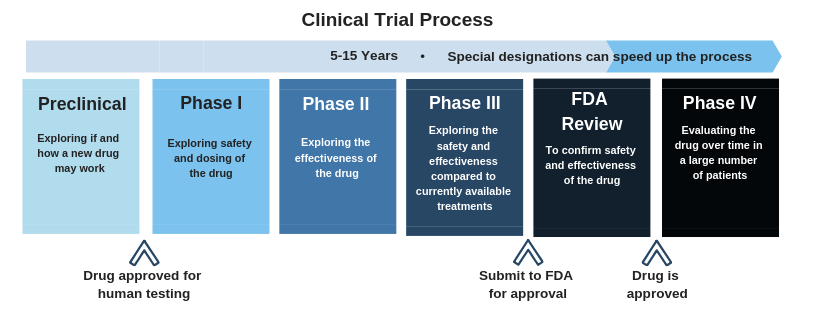 Click here
Click here
 We keep hearing that a combination of drugs will be necessary to cure HBV. What will these therapies look like?
We keep hearing that a combination of drugs will be necessary to cure HBV. What will these therapies look like?
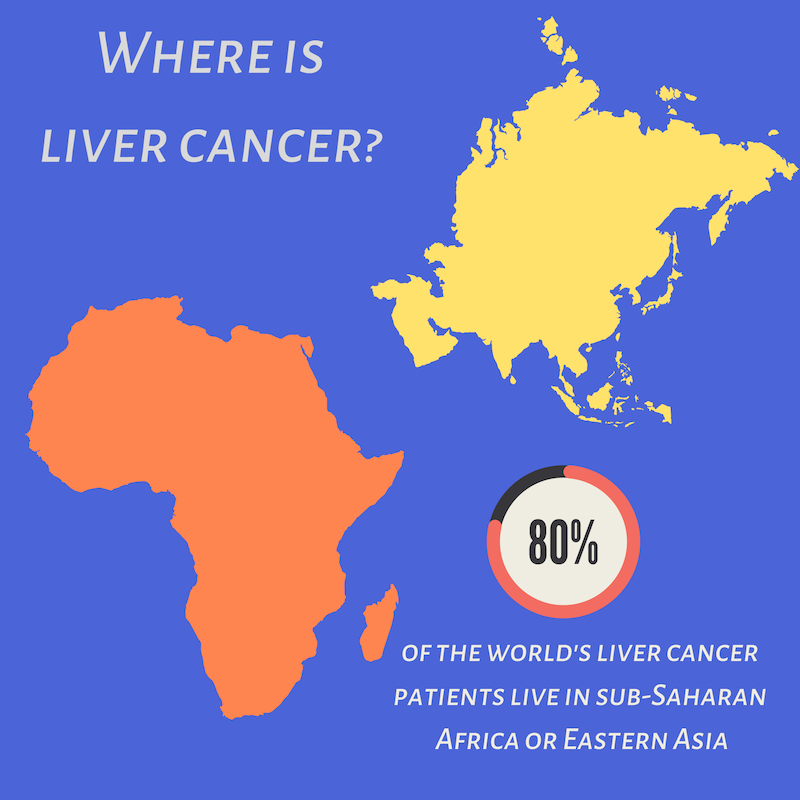 Liver cancer occurs when normal liver cells begin to gr
Liver cancer occurs when normal liver cells begin to gr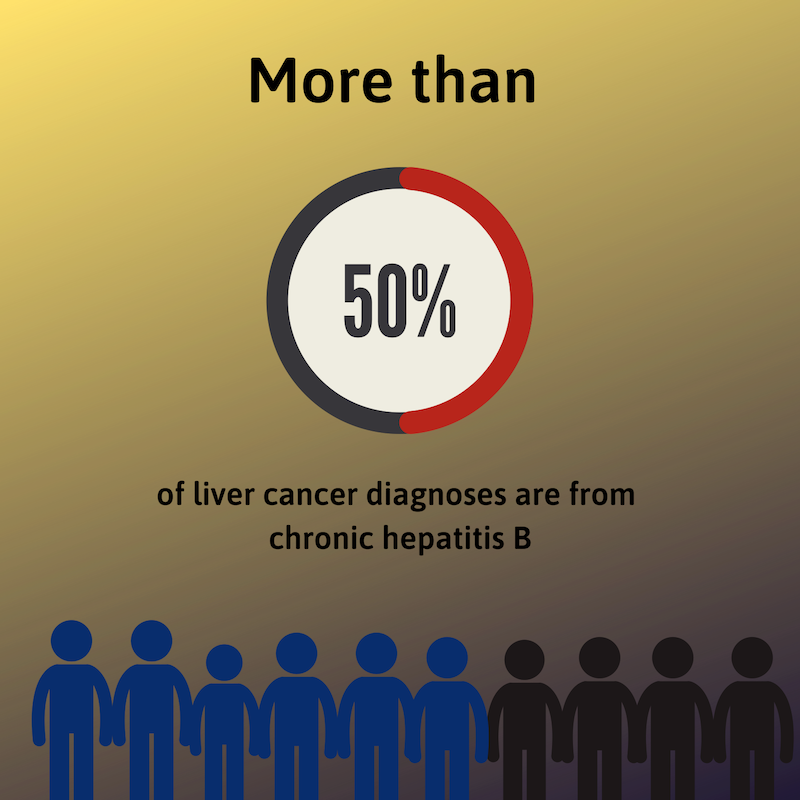
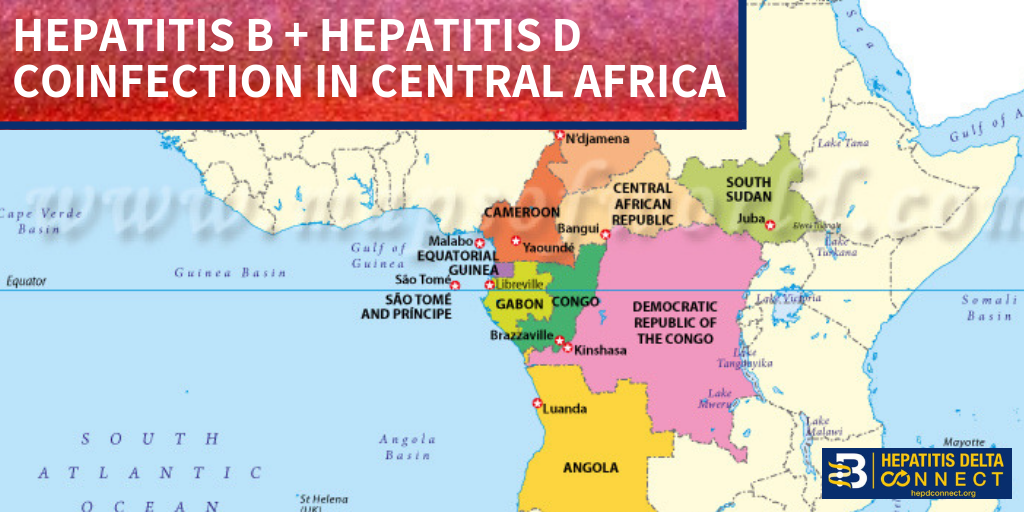
 Access (AFIA) program at the Multicultural Aids Coalition (MAC), the Coalition Against Hepatitis for People of African Origin (CHIPO), the New England AIDS Education and Training Center (NEAETC), and the Harvard University Center for AIDS Research (CFAR) in continuing the national fight for federal recognition of National African Immigrant and Refugee HIV and Hepatitis Awareness Day (NAIRAHHA).
Access (AFIA) program at the Multicultural Aids Coalition (MAC), the Coalition Against Hepatitis for People of African Origin (CHIPO), the New England AIDS Education and Training Center (NEAETC), and the Harvard University Center for AIDS Research (CFAR) in continuing the national fight for federal recognition of National African Immigrant and Refugee HIV and Hepatitis Awareness Day (NAIRAHHA).
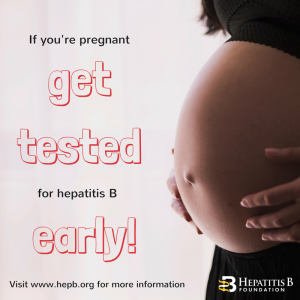 Around the world, the most common mode of hepatitis B transmission is from mother to child. Unfortunately, pregnant mothers who have hepatitis B can transmit the virus to their newborn during the delivery process. 90% of these HBV infected babies will progress to chronic infection putting them at increased risk of serious liver disease or liver cancer later in life.
Around the world, the most common mode of hepatitis B transmission is from mother to child. Unfortunately, pregnant mothers who have hepatitis B can transmit the virus to their newborn during the delivery process. 90% of these HBV infected babies will progress to chronic infection putting them at increased risk of serious liver disease or liver cancer later in life.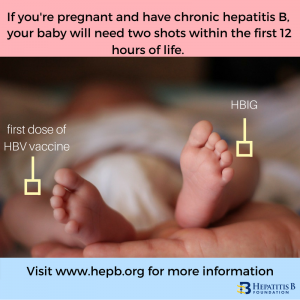 The
The 


 While
While  For more information on hepatitis B vaccine in babies or children, consult the “
For more information on hepatitis B vaccine in babies or children, consult the “